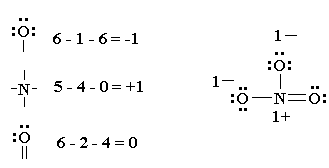Awe-Inspiring Examples Of Tips About How To Draw Dot Structures

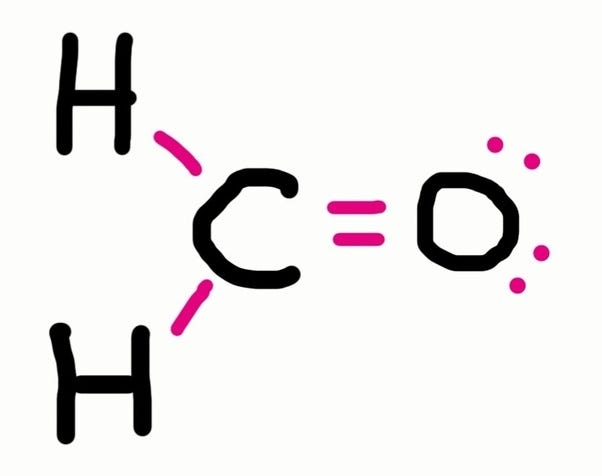
Shared pairs of electrons are drawn as lines between atoms,.
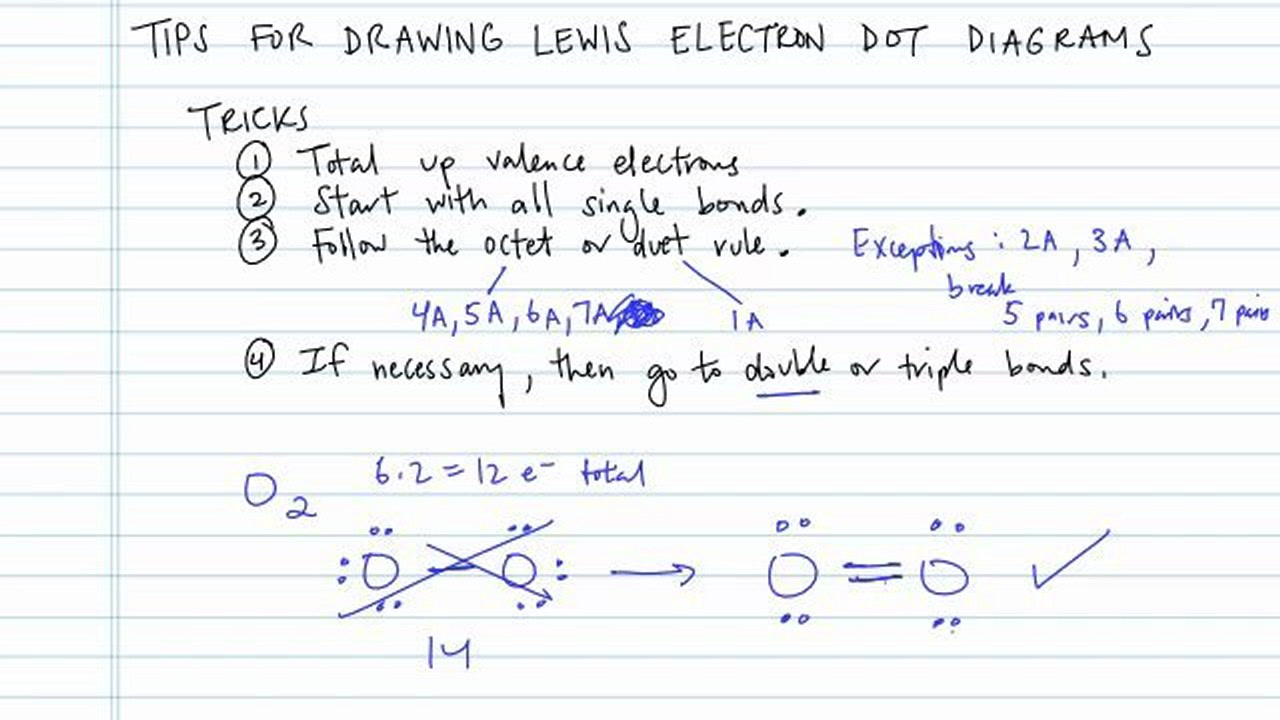
How to draw dot structures. There are several key steps for writing lewis structures: Steps to draw a lewis electron dot structure: Lewis structure is very important in chemistry, because they are used in.
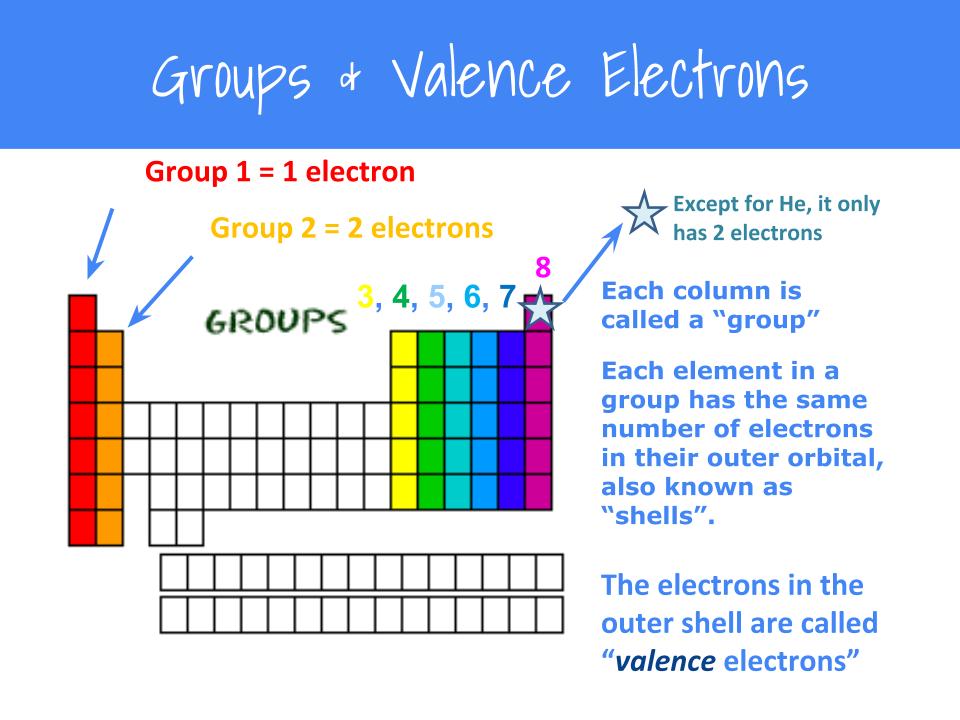
An electron dot structure, also known as a lewis dot formula, is a representation of the electrons found in the valence shell of an atom. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Valence electrons are the total number of electrons residing in the.
Now when you're drawing dot structures, you don't always have to do this step where you're drawing each individual atom and summing all of your valence electrons that way. Guidelines for drawing lewis dot structures. These instructions outline the kelter strategy to draw lewis structures for molecules.
Find the total number of valence electrons in this step, add up the total. It defines the nature of bond and position of atoms of the molecule which. Put the least electronegative atom in the center.
To draw the lewis dot structure of positively charged polyatomic ions subtract the positive charge value from the total valence electrons. Hydrogen (h) always goes outside. Find the total valence electrons for the molecule.
Find and count the total valence electrons. Electron dot structures or lewis dot formula can be drawn if the molecular formula of the compound is known. Put the least electronegative atom in the center.





/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)