Fun Tips About How To Write Empirical Formula

4) write the empirical formula:
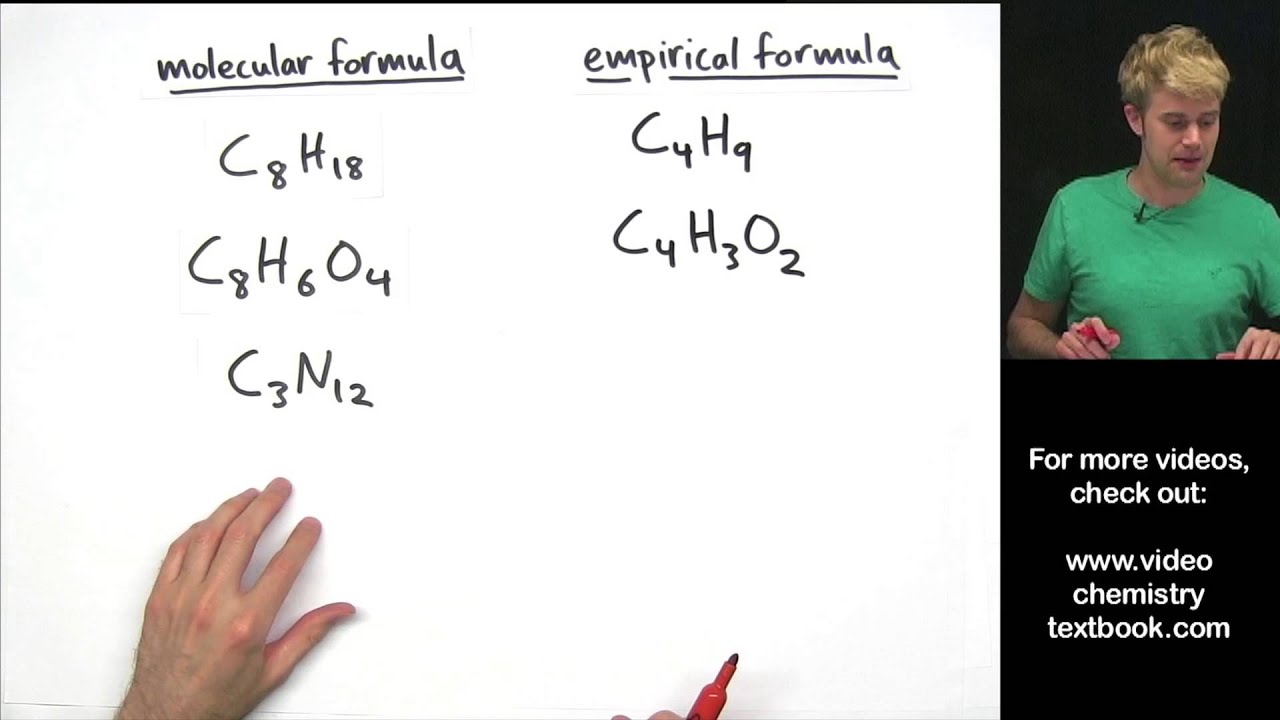
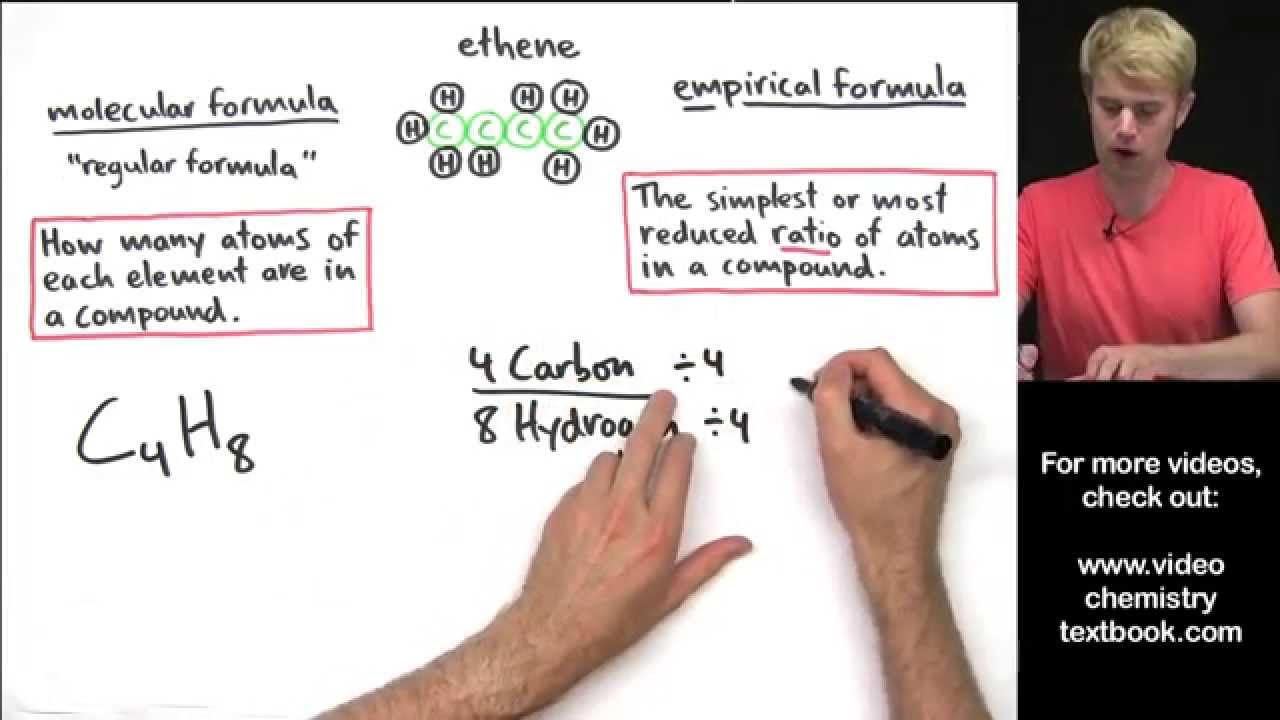
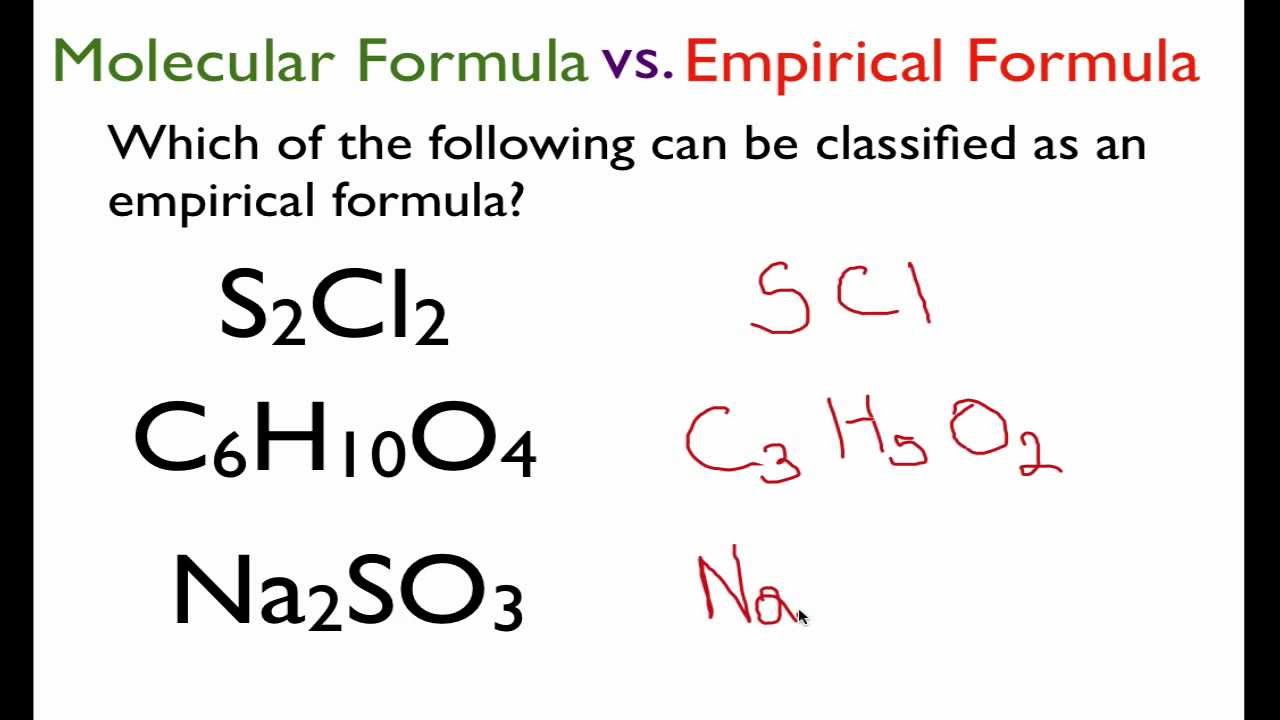
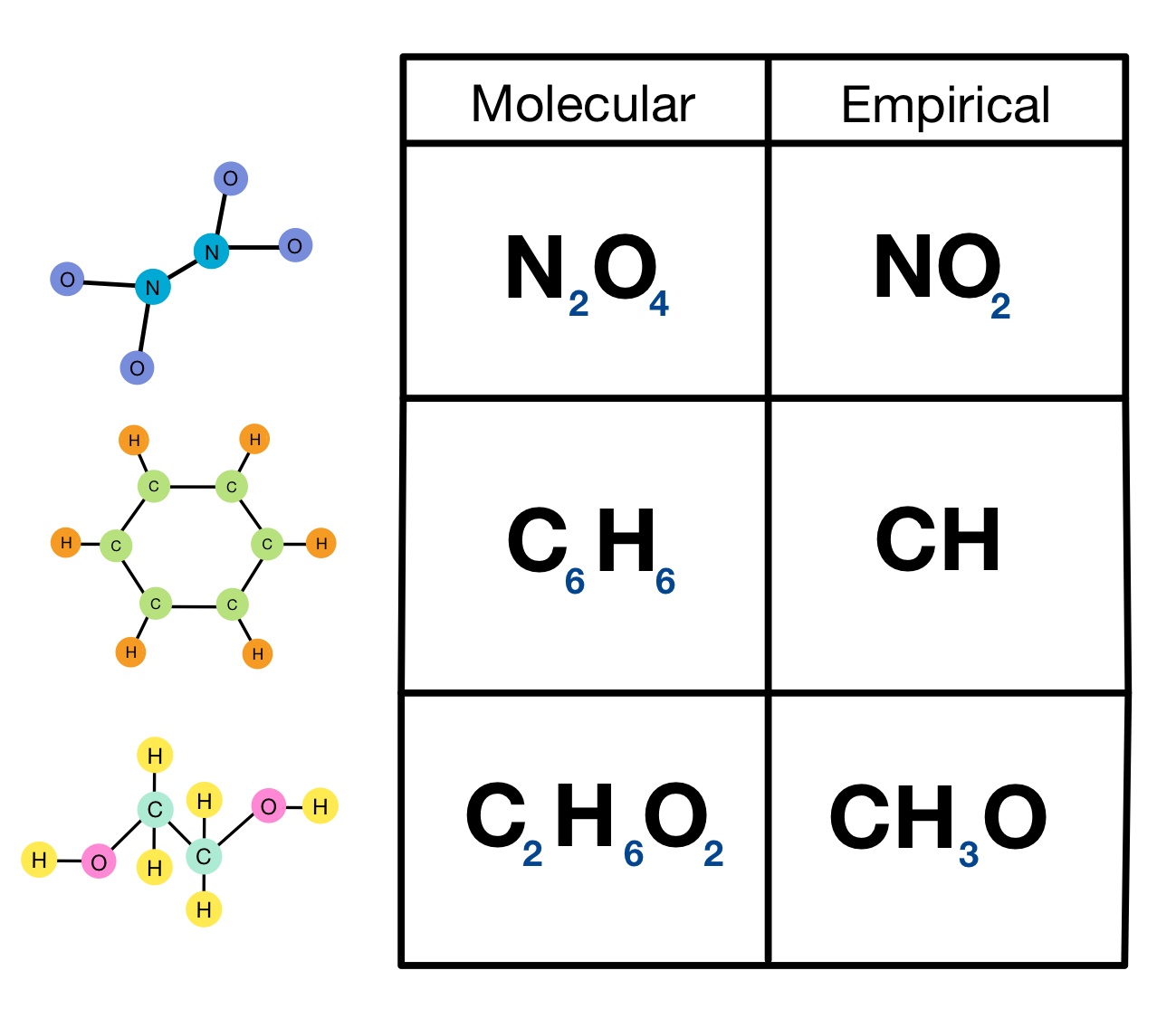
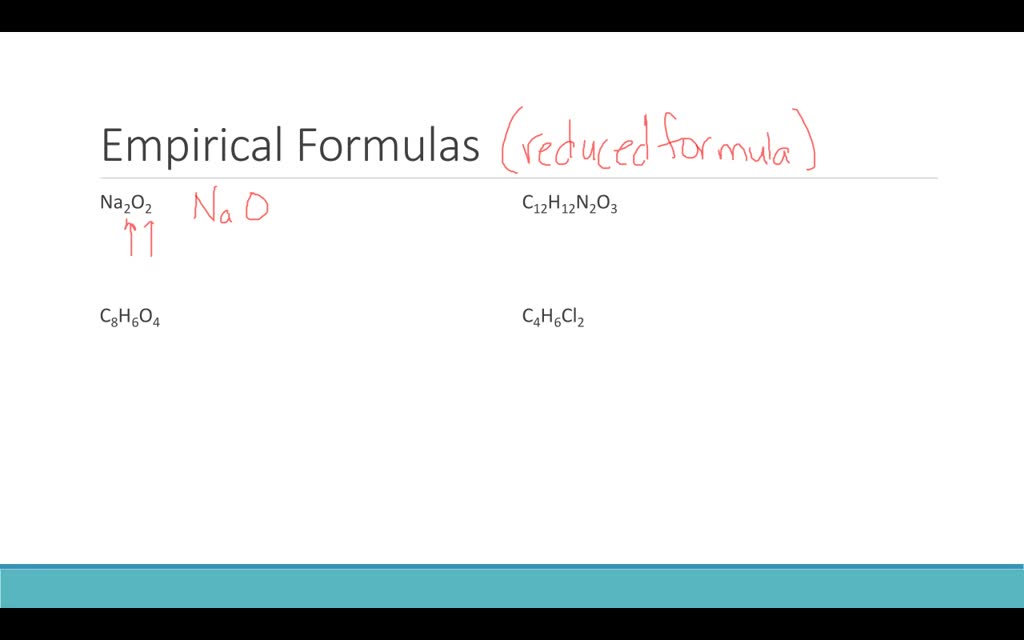
How to write empirical formula. The steps for determining a compound’s empirical formula are as follows:. There are three main types of chemical formulas: How to write the empirical formula:
7) use the scaling factor. For example, the empirical formula of a hydrocarbon is ch2 and its mr is 42. How to calculate empirical formula?
Divide the molar mass of the compound by the empirical formula mass. Multiply all the subscripts in the empirical. 5) compute the empirical formula weight: 32 + 16 + 16 = 64.
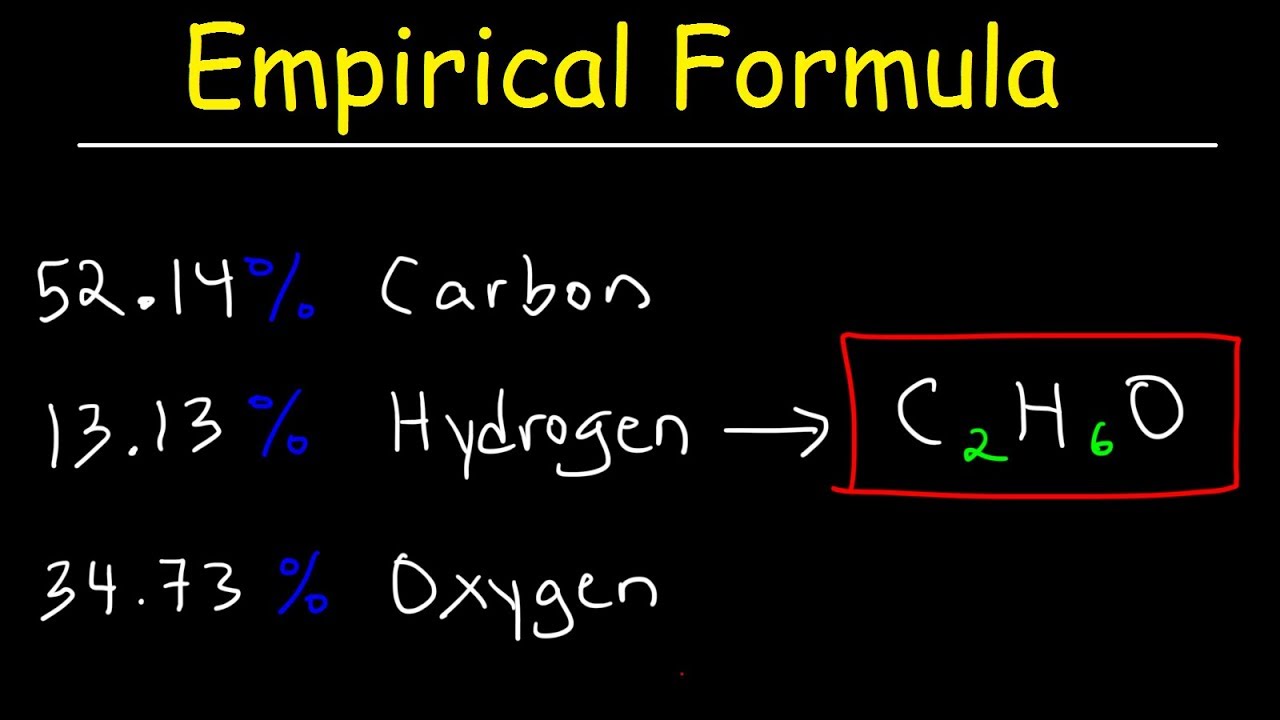
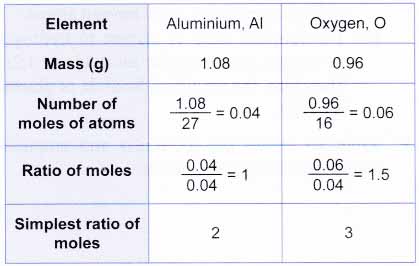
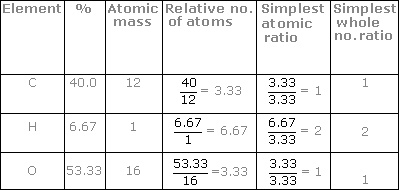
6) divide the molecule weight by the efw: 64.07 / 64 = 1. Order the elements according to the. The empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound.
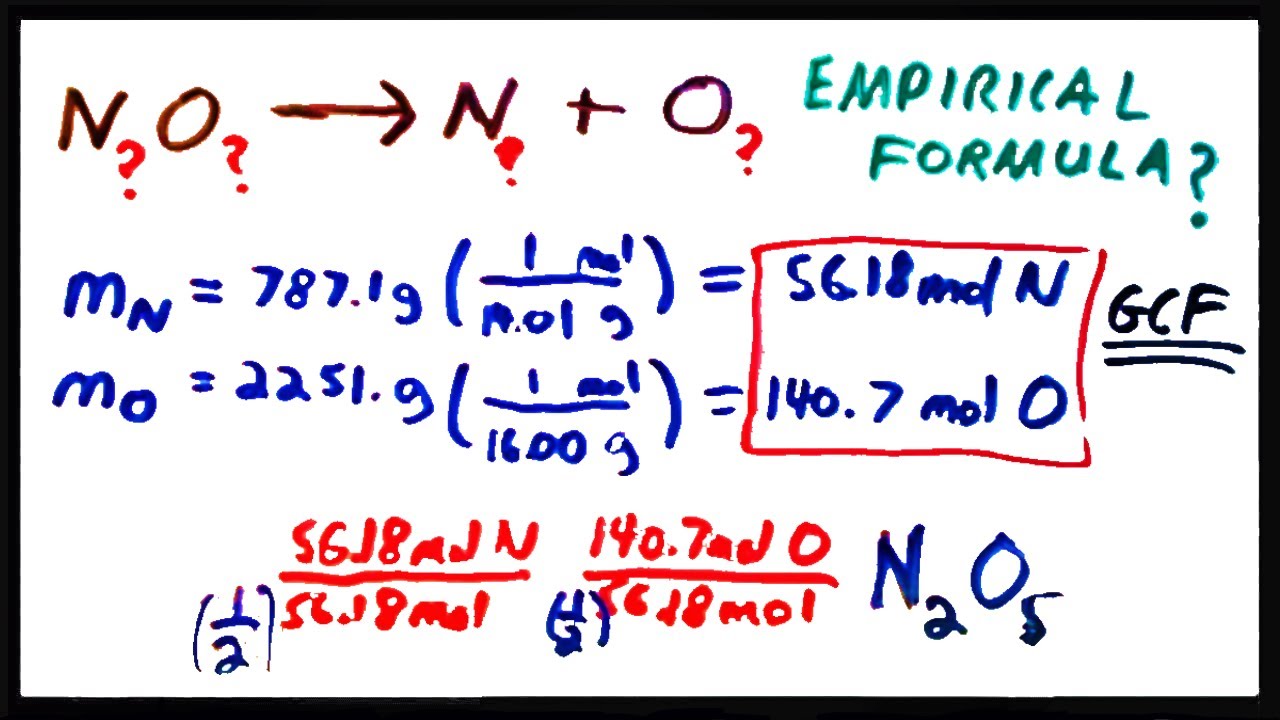
This video goes into detailed steps on how to find the empirical formula of a compound. Hooray for no more confusion!check out my new complete guide on empir. In the phosphorus oxide example the lowest number is 4.
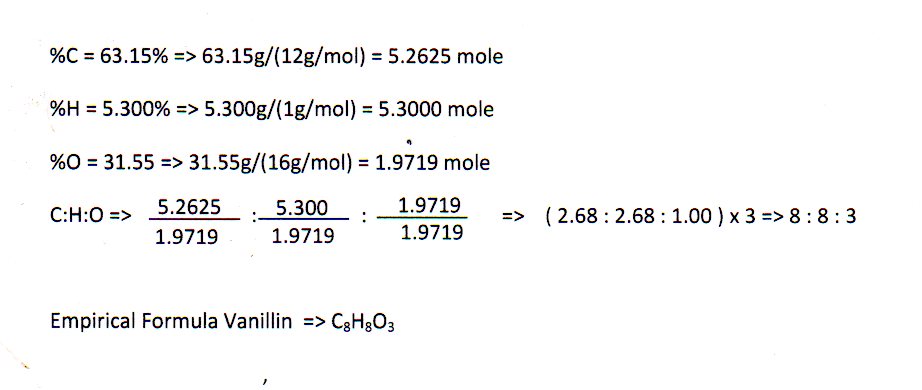
How to calculate empirical formula. Such essays are very difficult to write, because many are not interested in this and do not see the meaning of the text. An empirical formula can be calculated through chemical stoichiometry.